- Federal government urged to take action on judicial vacancies - March 22, 2024
- Canada Disability Benefit should not be a windfall for insurers - January 17, 2024
- Cost award like a ‘bomb going off in the world of LTD litigation’ - October 24, 2023
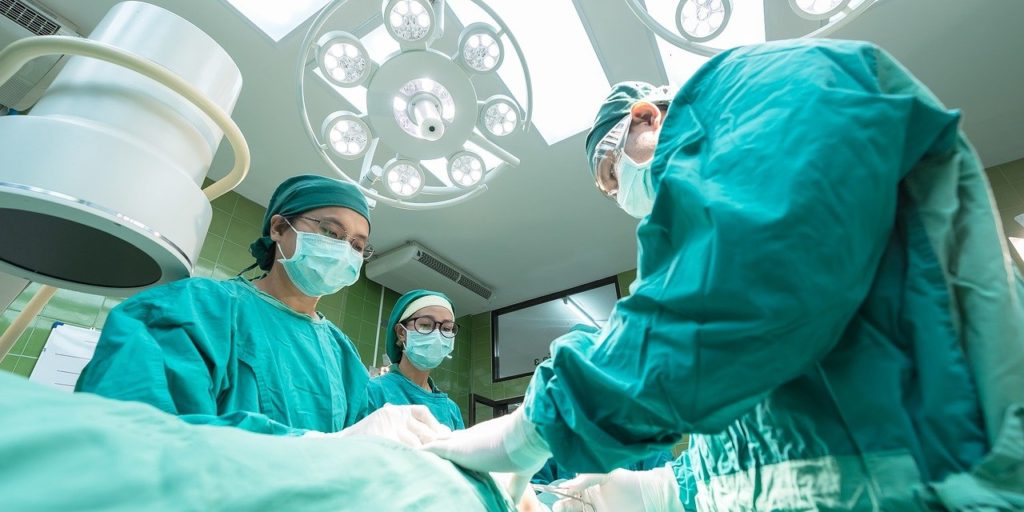
By Tony Poland, LegalMatters Staff • It is unlikely cases of medical device and medication litigation can be completely eliminated but class-action and mass tort lawsuits can help bring justice to victims and ultimately lead to improvements that benefit the public, says Barrie-area litigator Steve Rastin.
Rastin, senior counsel at Rastin Gluckstein, says more regulations or changes in testing and approval processes is not necessarily the answer.
“It would be great to head off instances of this type of litigation with more government oversight, but I do not believe there will ever be a way to completely prevent negative outcomes in the medical field under some circumstances,” he tells LegalMattersCanada.ca. “There can be many reasons for a bad outcome. For example, if the initial research is flawed, if the testing protocols were not proper or if approval happens too quickly. Even if a device is properly made or a medication is safe there is still the possibility of human error or a mistake in the manufacturing process.”
“It is actually a miracle that medical science does what it does because there are so many ways something can go wrong and that is why we need to be careful about pushing something new into general use until we check and triple-check that it is safe.”
Problems can arise if materials are substituted
Rastin, who handles class action and mass tort lawsuits along with Gluckstein Lawyers associate Jordan Assaraf, says there are cases where the prototype of a medical device works as it is intended but problems can arise if materials are substituted in the manufacturing process or if the device is improperly implanted.
He says Health Canada is responsible for regulatory approval of medications and medical devices.
“But part of the challenge is that while many of these testing processes are regulated by the government, many of the steps in the approval process are actually carried out by the medical companies,” says Rastin. “Of course, I am not alleging these companies are doing anything nefarious. There is no easy fix. But having more review of the process would be helpful.”
He says manufacturers of medical devices often will introduce the latest innovation to the public after it has been tested and then monitored its use in patients.
“However, you are not dealing with lawnmowers,” Rastin says. “These are medical devices that go inside people. When something goes wrong, people are left in excruciating pain or they may die. Some patients have to undergo multiple surgical procedures to correct the issue.
‘It might take years before the defect shows up’
“Part of the problem too, is sometimes it might take years before the defect shows up,” he adds. “We are constantly seeing issues arising, especially with respect to some joint replacement devices.”
Technology inevitably leads to a better way of doing things but due diligence is vital, Rastin says.
- Mass torts effective in ensuring access to justice for average plaintiff
- Slip and fall victims can be plowed under trying to sue municipalities
- As COVID issues increase, so will the need for litigation
“It’s an interesting debate about whether the technology we have is preferable or if it is better to use the technology being brought to market without the manufacturer or regulatory bodies being fully aware of some of the potential long-term risks,” he says.
Rastin says it can be argued “that efficiency is driven more by private enterprise than by the government” when introducing new medications or medical devices.
He cites the race to develop a COVID vaccine as an example.
‘There was tremendous pressure to rush a vaccine to market’
“There was tremendous pressure to rush a vaccine to market and, arguably, it has saved millions of lives,” Rastin explains. “But there were health risks and, in the rush, we might have put out a vaccine that was not optimal. What if the health risks of that vaccine had been worse?”
Medical advances may appear beneficial at first but later have devastating consequences, he says, pointing to Thalidomide, a medicatio given to women to treat morning sickness during pregnancy. However, its use was discontinued after tens of thousands of babies worldwide were born with a range of disabilities.
“Thalidomide was a medication that got through the regulatory process and was approved by our government for use in Canada,” Rastin says.
He says he believes class-action and mass tort litigation “unfortunately will going to be a growth area” due to medical device and medication issues.
“The reality is there is a role for class-action litigation counsel because it might not be prudent for an individual to sue on their own in many of these malpractice cases,” Rastin says. “If you had a hip replacement and needed another hip replacement, you might not find a lawyer to take on the manufacturer. It would be expensive to prove your case, requiring research and expert testimony. It might not be cost-effective.
“If mistakes are made in the development of new medications or medical devices, mass tort or class-action lawyers offer people an access to justice option that they might not otherwise have,” he adds.